
New Drug Designations - October 2023
Shots:
-
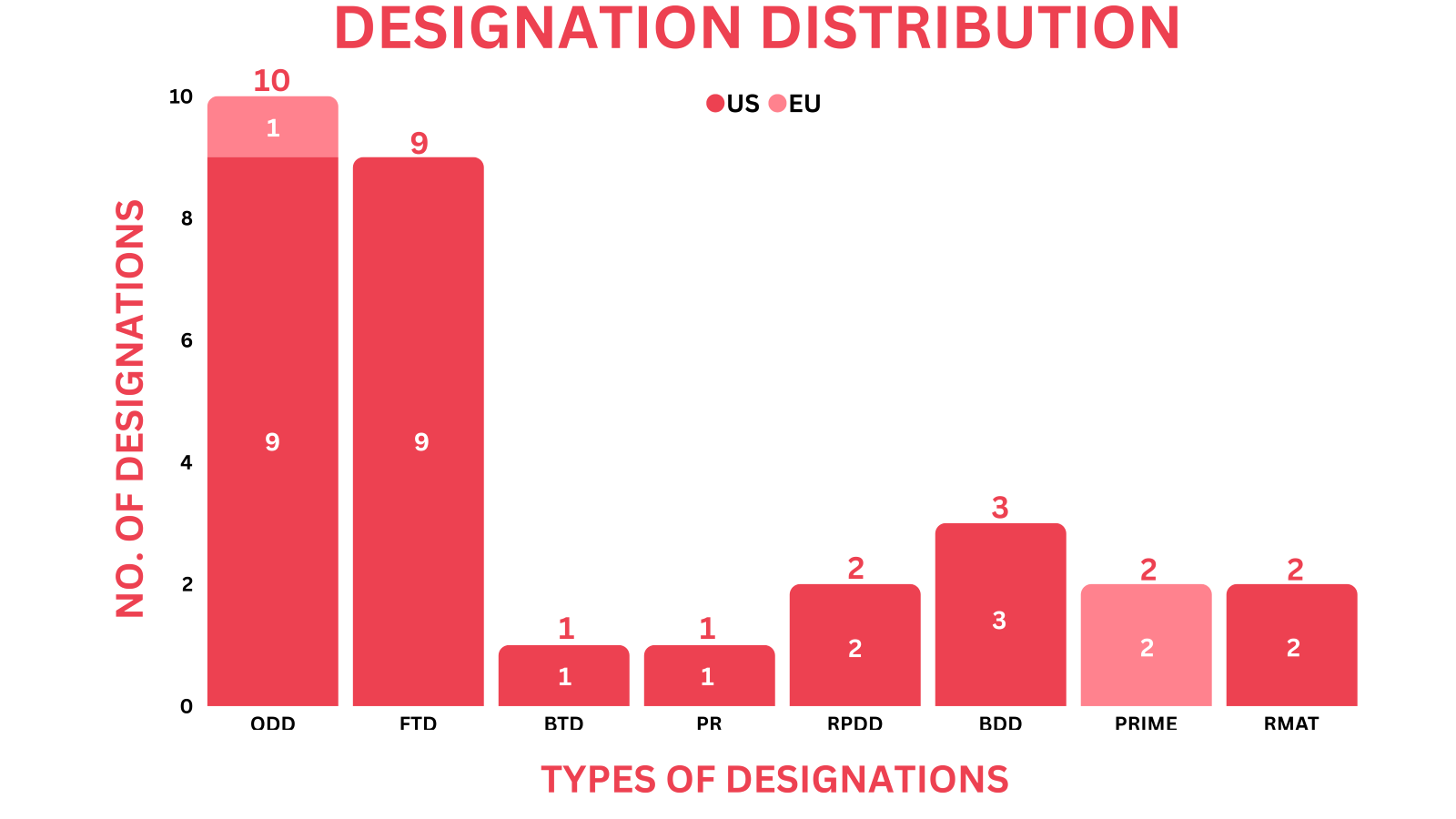
PharmaShots' designation report provides a concise overview of several drugs and their designations by the US FDA and the EMA. This month’s report includes 2 biological drugs, 12 small molecules, 10 cell and gene therapies, 2 vaccines, 1 peptide, 1 exosome-based therapy and 3 devices
-
HuidaGene Therapeutics’ HG204 CRISPR RNA-editing therapy, focused on the treatment of MECP2 duplication syndrome (MDS), is the drug to receive both RPDD and ODD from the US FDA
-
PharmaShots has compiled a list of a total of 28 drugs and 3 devices awarded with designations by multiple regulatory bodies in Oct 2023

SAGE-718

-
SAGE-718 is being investigated in multiple clinical studies incl. two PBO-controlled P-II studies and a P-III open-label safety study for HD-related cognitive impairment.
-
Additionally, P-II PBO-controlled studies are underway for mild cognitive impairment associated with Parkinson’s disease and mild cognitive impairment and mild dementia due to Alzheimer’s disease
-
SAGE-718 also received FTD from the US FDA for HD and ODD for HD by the EMA
FT011
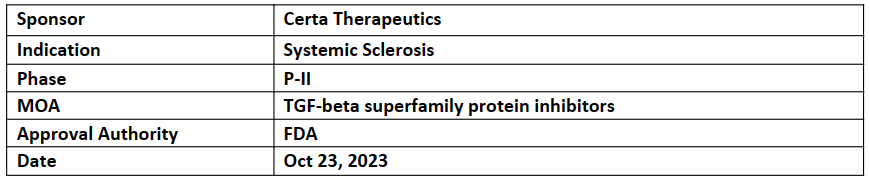
-
The ODD was based on the top-line results from the P-II study, presented at ACR Convergence 2023, which demonstrated clinically meaningful improvement in 60% (p=0.019 vs PBO) of patients treated with FT011 (400 mg) and 20% of patients in the FT011 (200 mg) group vs 10% in the PBO group
-
Three patients in the combined FT011 groups attained a maximum CRISS score of 1.0, indicating a higher likelihood of clinical improvement
-
The safety profile showed that the drug was safe and well tolerated without differences in AE rates between the treatment arms. Neither serious AEs nor any AEs resulting in drug interruption, withdrawal, or discontinuation were reported
-
FT011 is a novel, FIC, orally administered therapy for the treatment of chronic fibrosis in multiple organs advancing through clinical development for scleroderma (systemic sclerosis)
UV1
-
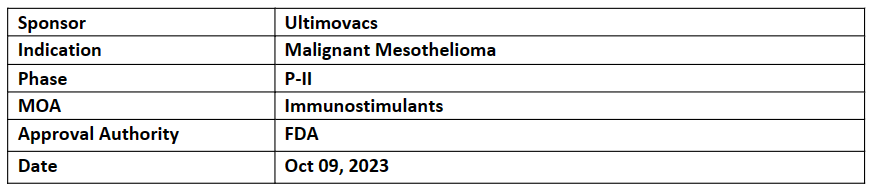
FDA granted ODD to UV1 vaccine for the treatment of mesothelioma. The decision is based on the initial data received from P-II trial (NIPU)
-
In P-II (NIPU) trial UV1 vaccine in combination with Ipilimumab + nivolumab assessed as 2L treatment in 118 patients across Australia, Denmark, Norway, Spain, and Sweden. FPI in Jun’20 and LPI in Jan’23. This study was sponsored by University of Oslo in collaboration with BMS and Ultimovacs
-
Human telomerase (hTERT) enzyme is the target of the therapeutic cancer vaccine UV1, which generate an immune response against it. In 85–90% of all cancer stages of the disease. UV1 vaccine already received ODD from FDA for Melanoma patients in Dec’21
Risvodetinib (IkT-148009)

-
Inhibikase’s Risvodetinib (IkT-148009) has been granted ODD by the FDA for the treatment of Multiple System Atrophy (MSA)
-
In Mar'23, Inhibikase received IND clearance to plan P-II study in MSA. Preclinical results were presented in Aug’23 showing risvodetinib could be therapeutically active in models of disease
-
Biologics are intended for the prevention, diagnosis or treatment of diseases that affect 200,000 people in the US. Multiple System Atrophy (MSA) is a rare disease with 3.6 to 4.9 cases per 100,000 people in the US Population. It affects equally both the genders
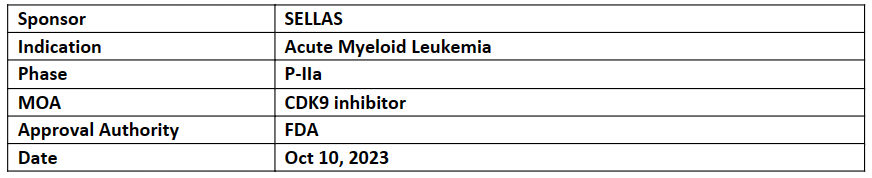
SLS009 (formerly GFH009)
-
The ODD was based on the results from P-I trial which showed the following:
-
Efficacy results: anti-tumor activity of up to 77.3% bone marrow blast reduction, durable CR with no MRD, 24-hour concentrations in peripheral blood after the first infusion, with IC90 concentrations resulting in up to 97% cancer cell killing and MCL1 and MYC suppression in peripheral blood in 97% (66/68) patients
-
Safety results: There were no DLTs and higher grade non-hematologic toxicities of any kind. Hematologic toxicities of short duration, reversible and difficult to determine in patients with hematologic cancers were observed
-
The company is investigating SLS009 (selective CDK9 inh.) in an open-label, single-arm, multi-center P-IIa trial to treat r/r AML for its safety, tolerability, and efficacy at two dose levels (qw, 45 mg and at the RP2D, 60 mg) in combination with azacitidine and venetoclax. Topline results are anticipated by YE’23
-
SELLAS licensed SLS009 from GenFleet Therapeutics for all therapeutic and diagnostic uses worldwide accept Greater China
FG001

-
FDA granted ODD to FG001 (diagnostic agent) for the management of malignant glioma. The designation applies for use of FG001 in the most aggressive gliomas (WHO grades III-IV)
-
FG001 is a fluorophore targeting uPAR, fluorophore has the same spectral specifications as already approved indocyanine green administered intravenously prior surgery.
-
FluoGuide is assessing the effects of FG001 in a randomized, P-llb trial (FG001-CT-001) in guiding surgery of patients with aggressive brain cancer vs FG001’s effect to 5-ALA and white light. Results from this study are expected in late Nov’23
-
P-I/IIa study showed highly promising results in the same indication, where 100% of the biopsies from patients treated with FG001 illuminated cancer.
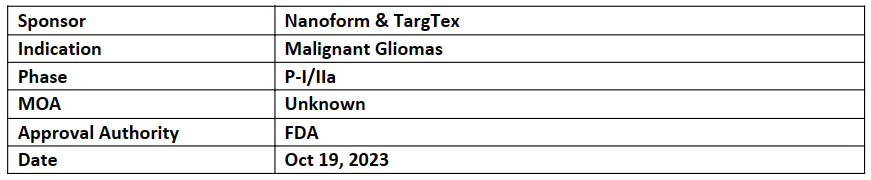
TTX101
-
TargTex received ODD for its Novel hydrogel-based targeted nano-formulation therapy TTX101 which is being developed to treat malignant gliomas. This ODD is based on the results received from the preclinical study that showed the potential for patients with glioblastoma or other aggressive CNS tumors, either alone or in combination with other therapies
-
TargTex is developing TTX101 in collaboration with Nanoform utilizing their hydrogel nanoformulation technique which will enable a 200-fold increase in drug load for TTX101
-
TargTex is raising funds move the advance the TTX101 to clinic and planning a P-I/IIa trial in recurrent glioblastoma (GBM) patients across the US and EU, in which nanoformed TTX101 is applied as adjunct to surgery after tumor excision
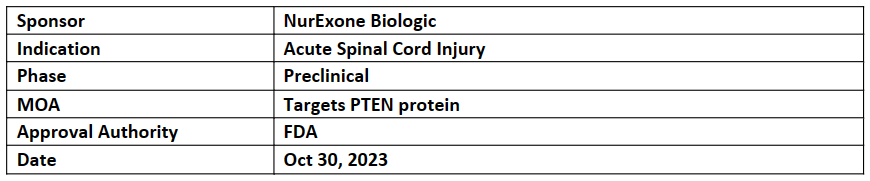
ExoPTEN therapy
-
ExoPTEN therapy, based on the ExoTherapy platform, was awarded ODD by the US FDA for the treatment of acute spinal cord injury
-
The designation involves utilizing MSC-derived EVs loaded with siRNA to target PTEN protein for acute spinal cord injury in the development of the company's ExoPTEN drug
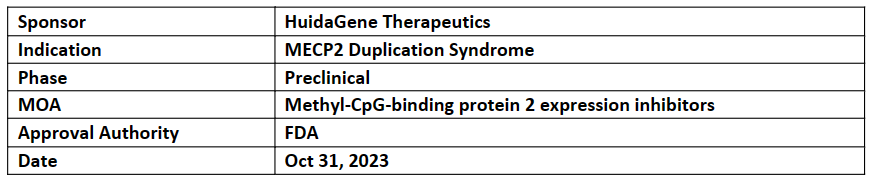
HG204
-
HG204 (CRISPR RNA-editing therapy), has received both RPD and ODD designations from the US FDA for the treatment of MECP2 duplication syndrome (MDS), a rare and fatal childhood neurodevelopmental disorder with no available treatment
-
HG204 (AAV-hfCas13Y-gMECP2) is designed using a single AAV vector delivering a novel CRISPR/high-fidelity Cas13Y (hfCas13Y) and gRNAs targeting MECP2 (gMECP2)
LSTA1
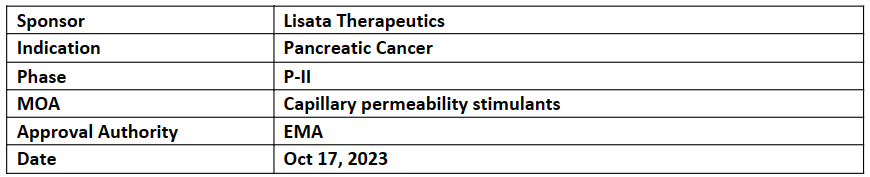
-
LSTA1 has shown meaningful safety profile, tolerability and activity in the studies which can enhance the delivery of SoC CT for pancreatic cancer
-
LSTA1, with various anti-cancer regimens, is being investigated in multiple ongoing and planned trials conducted worldwide in solid tumors incl. pancreatic cancer
-
LSTA1 activates a novel uptake pathway allowing co-administered or tethered anti-cancer drugs for effective solid tumor penetration

AGMB-129
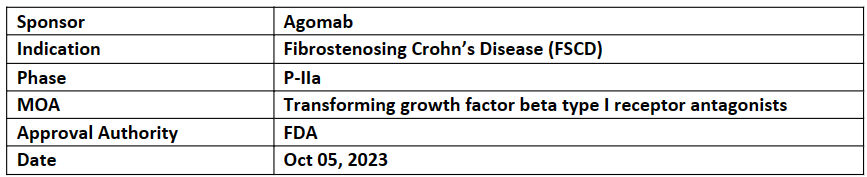
-
AGMB-129, an oral gastro-intestinal (GI)-restricted ALK5 (TGF-RI or ALK5) inhibitor small molecule, presently being evaluated in 36 patients with symptomatic FSCD as part of Agomab's STENOVA P-IIa clinical trial
-
1EPs safety and tolerability of AGMB-129 in patients with FSCD. The PK and target engagement at the location of the ileal strictures are examples of 2EPs
-
Intestinal strictures are the primary cause of bowel surgery, induce the formation of fistulae, and cause severe obstructive symptoms
SurVaxM

-
The US FDA granted FTD to MimiVax's SurVaxM vaccine newly diagnosed glioblastoma (nGBM) patients
-
MimiVax is assessing SurVaxM in a randomized, blinded placebo-controlled P-IIb (SURVIVE) study in nGBM patient [NCT05163080] recruiting at 11 sites across the US
-
Positive data from P-IIa trial published in the Journal of Clinical Oncology, 2-yr survival was 51% and 3-yr survival 41%. The mOS achieved 25.9 mos with nGBM vs SoC.
-
SurVaxM after FTD may be eligible for accelerated approval and priority review during NDA
AVB-001
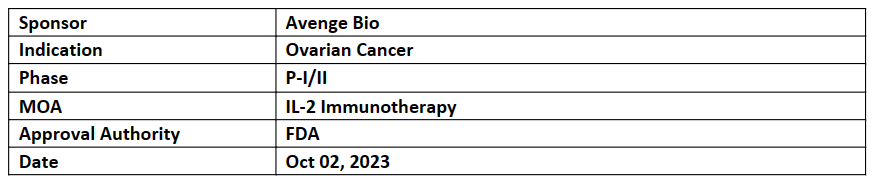
-
The USFDA has given FTD for AVB-001 for treatment of patients with relapsed resistant/refractory ovarian cancer (including platinum-resistant, high grade serous adenocarcinoma of the ovary, primary peritoneum or fallopian tube)
-
In Jan’23, Avenge initiated an open-label, FIH, P-I/II (NCT05538624) to evaluate safety and efficacy of AVB-001. In addition to ovarian cancer, Avenge Bio is also assessing other peritoneal malignancies and pleural cancers. P-II dose expansion study expected to initiate in H1'24
-
AVB-001 is an encapsulated engineered product to produce native human interleukin-2 (hIL-2) and is delivered intraperitoneally (IP) to patients. AVB-001 is developed using a proprietary technology LOCOcyteTM allogeneic cell-based immunotherapy platform.
ANPD001
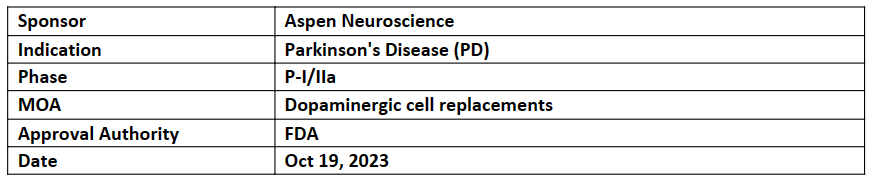
-
Aspen intends to start P- I/IIa FIH, multicenter, dose-escalation study to assess the safety, tolerability and preliminary efficacy of ANPD001 for the treatment of moderate-to-severe Parkinson's disease (PD) in the US
-
ANPD001 is an autologous cell therapy used to treat PD and enhance motor function by replacing depleting dopamine neurons
IMPT-514
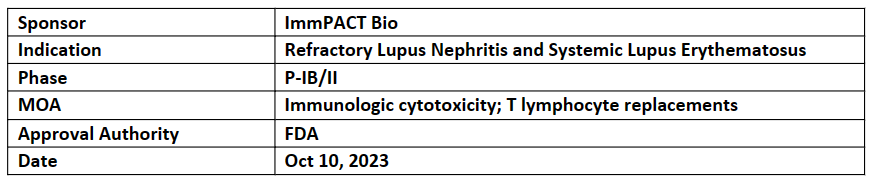
-
The US FDA has granted FTD to the company’s IMPT-514 for the treatment of patients with active refractory LN and SLE. The first patient dosing in the P-Ib/II trial for the treatment of active, refractory SLE is expected in early 2024
-
Additionally, IMPT-514 showed potent elimination of autologous B cells and a moderate cytokine profile in preclinical studies & was successfully and efficiently manufactured from heavily immunosuppressed patients with LN and SLE
-
IMPT-514 is a CD19/CD20-targeting CAR T-cell therapy that uses a potent bispecific CAR and a 4-1BB costimulatory domain
-
If company meet the relevant criteria, the FDA may consider reviewing portions of a marketing application before submission of the complete application
-
IMPT-514 is a CD19/CD20-targeting CAR T-cell therapies for treating patients with active lupus nephritis and systemic lupus
-
Phase Ib/II dose escalation study in participants with active, refractory Systemic Lupus Erythematosus (SLE) expected in early 20
ONCT-534
-
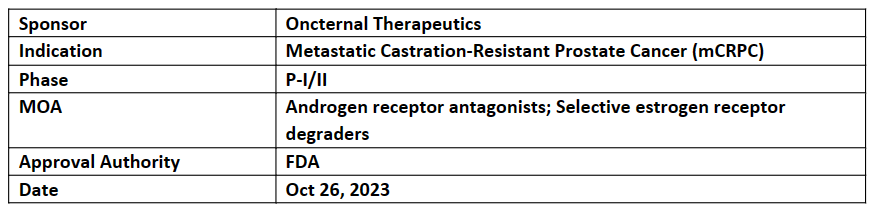
The US FDA has granted FTD to ONCT-534, its novel dual-acting androgen receptor inhibitor for the treatment of patients with r/r mCRPC resistant to approved androgen receptor pathway inhibitors (ARPIs)
-
The P-I/II study (ONCT-534-101) evaluating the safety and tolerability, PK, and preliminary anti-tumor activity of ONCT-534 in patients with mCRPC who have relapsed or are refractory to approved ARPIs incl. enzalutamide, abiraterone, apalutamide and daralutamide
-
The P-II of the study will begin to further investigate the safety and preliminary anticancer activity of ONCT-534 to choose optimal dose, after the assessment of the drug's tolerability, safety, and preliminary antitumor activity in P-I
-
The company closely working with the US FDA, investigators, and industry collaborators to bring ONCT-534 to patients shortly. Preclinical studies showed the activity in prostate cancer models against unmutated AR & against multiple mutations, incl. AR amplification, mutations in the AR LBD, and splice variants with loss of the AR LBD
SRP-001
-
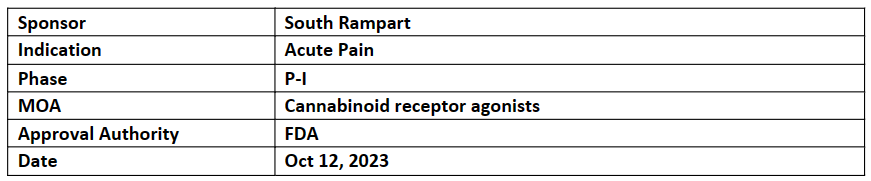
US FDA has granted FTD to SRP-001 for the treatment of acute pain
-
South Rampart Pharma is assessing SRP-001 (Oral) in the P-I MAD study for its safety, tolerability, and PK/PD in HVs, with 1EPs incl. safety and tolerability assessments and dosed first patient in Aug’23. The trial is anticipated to conclude in Q4’23
-
SRP-001 is a novel, FIC non-opioid analgesic that activates pain signaling pathways in the midbrain's periaqueductal grey (PAG) region without liver and kidney toxicities
BI 764532
-
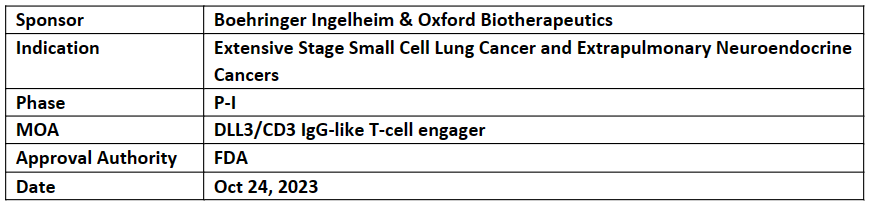
US FDA granted FTD to BI 764532 for 2L+ ES-SCLC (incl. PtCT), and of advanced or metastatic 1L+ epNEC (incl. PtCT) patients
-
The FTD was based on the P-I FIH, dose escalation trial results, presented at ASCO’23, which showed favorable safety and early efficacy in patients with DLL3-positive ES-SCLC and epNECs
-
BI 764532, DLL3/CD3 IgG-like T-cell engager, is based on the OBT’s proprietary OGAP drug discovery platform and Boehringer Ingelheim’s longstanding expertise
ONCT-534
-
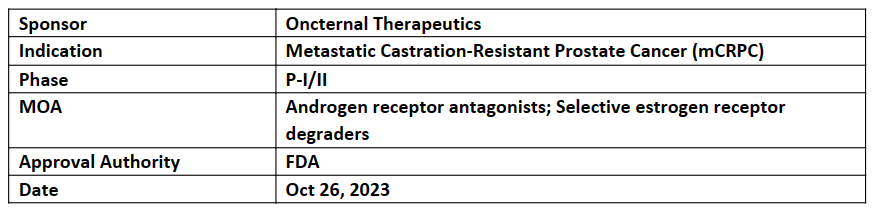
The US FDA has granted FTD to ONCT-534, DAARI inhibitor for the treatment of patients mCRPC with the help of ARPIs, After the result with ARPI incl. enzalutamide or abiraterone. They are going to work with FDA and collaboration to bring quickly ONCT-534
-
The N- terminal and LBD both interact with ONCT-534 as well as inhibiting the cell growth and inducing AR degradation
-
Prostate Cancer models shown activity in pre-clinical studies against both unmutated AR and against multiple mutations
CSX-1004
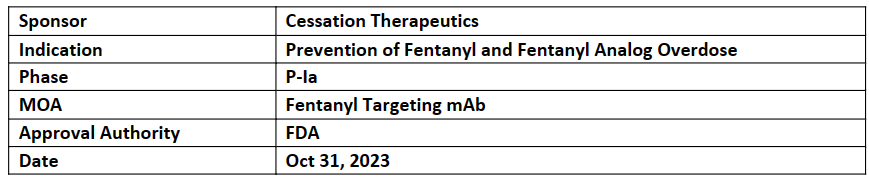
-
The FTD was influenced by data from nonhuman primate studies, which showed that a single dose of CSX-1004 can effectively prevent the life-threatening respiratory depressant effects of high fentanyl doses for a duration of up to 28 days
-
The company has initiated a P-Ia, FIH trial (NCT06005402) to assess the safety, tolerability, and PK of CSX-1004 in healthy volunteers
-
CSX-1004, a human IgG1 mAb, targets fentanyl and its analogs. It operates by sequestering these molecules upon entering the bloodstream, neutralizing them before reaching the brain. This prevents their harmful effects effectively

Furmonertinib
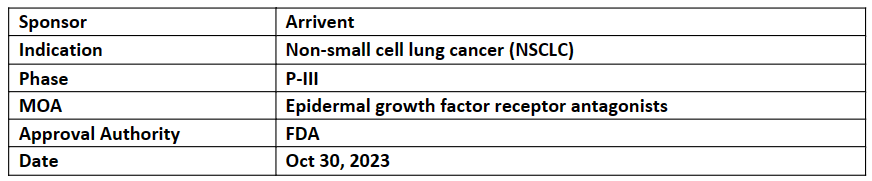
-
The FAVOUR trial was granted by BDA, P-1b evaluating the efficacy and safety of furmonertinib in patients intended to treat with the help of drugs serious life-threatening conditions
-
The ongoing P-III FURVENT trial of furmonertinib in 1L NSCLC patient with EGFR exon 20 insertion mutation as well as those are classical (exon 19 deletion and L858R)

Mavorixafor
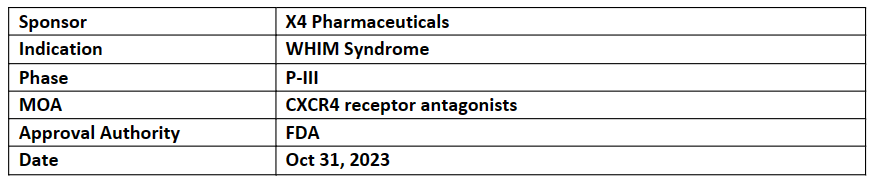
-
US FDA has accepted and granted priority review to NDA of mavorixafor for the treatment of individuals aged 12 + years with WHIM (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) syndrome, a rare, primary immunodeficiency with the expected PDUFA on Apr 30, 2024
-
The NDA for mavorixafor was based on positive results from the global P-III (4WHIM) study for WHIM syndrome. The trial met its 1EP (TAT-ANC) vs PBO (p<0.0001), demonstrated favorable safety, and showcased reductions in infection rates, severity, and duration
-
Mavorixafor, an investigational small-molecule antagonist of the CXCR4 receptor, is in development as a QD oral therapy for WHIM syndrome and specific chronic neutropenic disorders
Marnetegragene Autotemcel (RP-L201)
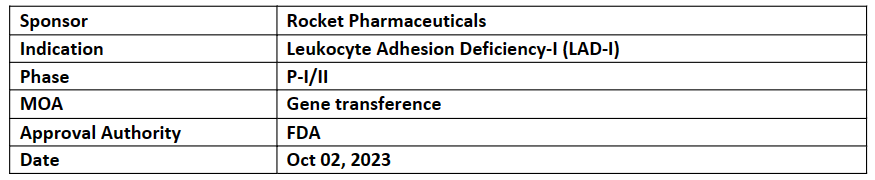
-
FDA granted Priority Review for the BLA for RP-L201 for severe LAD-I with expected PDUFA on Mar 31, 2024
-
Priority Review is based on the P-II study with follow-up data of 12-24mos. 100% patients achieved overall survival (n=9) and resolution of LAD-I related skin rash and restorated wound repair function with no TEAEs reported
-
US FDA has already granted RMAT, Rare Pediatric, Orphan Drug, and Fast Track designations for RP-L201; and PRIME, Advanced Therapy Medicinal Product, and ODD from EMA

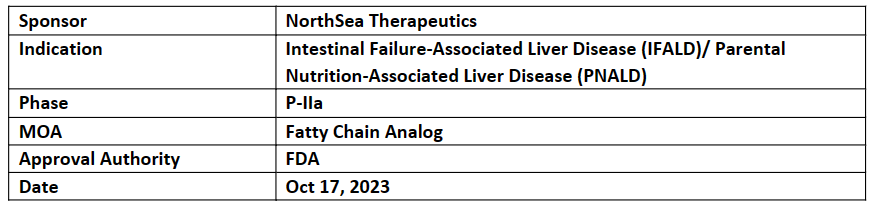
SEFA-6179
-
The company has successfully completed the P-I FIH study of SEFA-6179 for intestinal failure-associated liver disease (IFALD), the results of which showed that the drug was well-tolerated up to 1,000 mg, qd for 14 days and TEAEs were mild or moderate in severity
-
NorthSea is currently evaluating SEFA-6179 (novel, oral, fully synthetic medium chain fatty acid analog) in the P-IIa PoC study for IFALD. The topline data is anticipated in 2025. NorthSea Tx plans to initiate pediatric study soon
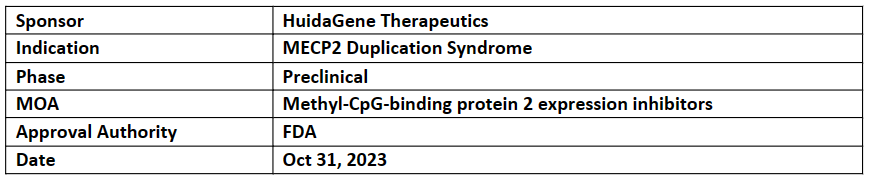
HG204
-
HG204 (CRISPR RNA-editing therapy), has received both RPD and ODD designations from the US FDA for the treatment of MECP2 duplication syndrome (MDS), a rare and fatal childhood neurodevelopmental disorder with no available treatment
-
HG204 (AAV-hfCas13Y-gMECP2) is designed using a single AAV vector delivering a novel CRISPR/high-fidelity Cas13Y (hfCas13Y) and gRNAs targeting MECP2 (gMECP2)

Selective Cytopheretic Device

-
FDA issued BDD for its patented, FIC, cell directed Selective Cytopheretic Device (SCD) for ICU patients with acute kidney injury (AKI) and acute or chronic liver failure. This is the 3rd BDD given by FDA to SeaStar Medical for the SCD device, and is expected to expedite the clinical development and regulatory review of the SCD for use in this patient population
-
University of Michigan initiated pilot study assessing treatment with the SCD in two patients with type 1 hepatorenal syndrome. Both the patients have shown positive response where:
-
Patient one has hepatorenal syndrome due to acute alcoholic hepatitis was alive at day 90 after seven days of SCD treatment and undergoing liver transplantation evaluation,
-
Patient two has hepatorenal syndrome due to NASH had a successful liver transplantation 6 days after SCD therapy ended
-
Results from both the studies have been published in American Society for Artificial Internal Organs Journal Aug 2023
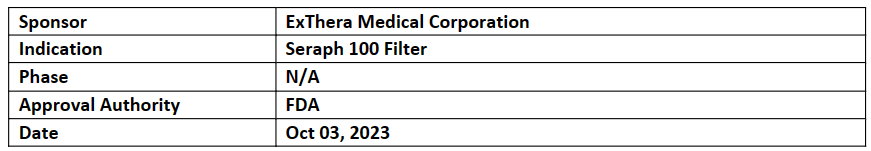
Seraph 100 Microbind
-
Exthera received multiple BDD for the Seraph 100 Microbind Affinity Blood Filter (Seraph 100). The Seraph 100 is a patented blood filter containing a biomimetic surface, which closely replicates the human circulatory system that removes pathogens that could otherwise overwhelm a patient immune system
-
Serpah 100 is being assessed in the PURIFY RCT bloodstream infection clinical study, which is an interventional multicenter randomized controlled trial, sponsored by the DOD
-
Seraph can be used in hospitals, clinics, or field hospitals to address nosocomial and community-acquired infections as well as those caused by battlefield wounds and pandemics. The Seraph 100 attained CE Mark and is commercially available in the EU. The Seraph 100 has FDA Emergency Use Authorization (EUA) for treatment of COVID-19 in the USA
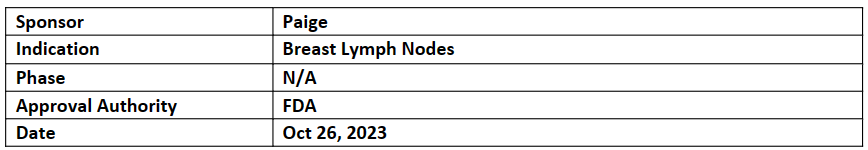
Paige Lymph Node
-
The FDA has designated the Paige Lymph Node as a Breakthrough Device, making it the first AI application of its kind. The Potential to provide more accurate and quickly identifying treatment for life-threatening even tiny lymph node metastases
-
The software for the in vitro diagnostic medical device known as Paige Lymph Node was developed using a deep learning model that was trained on more than 32,000 digitally scanned hematoxylin and eosin (H&E) lymph node slides

ANX007

-
Annexon received PRIME designation for ANX007 for Geography Atrophy secondary to AMD. It was granted based on the P-II (ARCHER) study indicating a statistically significant, durable, and dose-dependent preservation of visual function in GA patients & preclinical data supporting ANX007's protective effect against photoreceptor damage
-
Topline data, revealed in May 2023 and presented at the ASRS in July 2023, demonstrated ANX007 yielded statistically significant, time and dose-dependent protection against vision loss in patients with geographic atrophy measured by using BCVA ≥15-letter loss
-
ANX007, a fragment antigen-binding (Fab) antibody, designed to selectively inhibit C1q, the initiating molecule of the classical complement pathway and a significant contributor to neurodegeneration
NTLA-2002
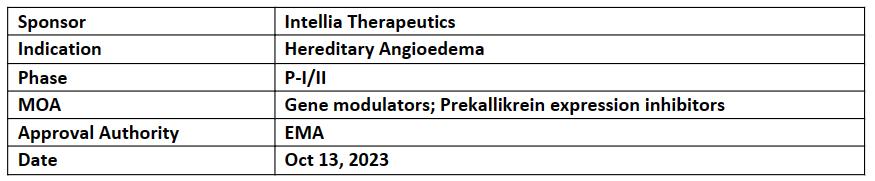
-
EMA granted PRIME designation to NTLA-2002 (in-vivo CRISPR therapy) for the treatment of hereditary angioedema (HAE) patients
-
Designation was granted based on the positive interim results of the P-I part of the P-I/II study in HAE. Of 10 patients dosed in P-I, after single dose reduction in monthly attack rate observed to be 95%. Median duration of follow-up was 9mos (range 5.6-14.1mos). All the 3 level of doses are safe and well tolerated with mild AEs
-
Before PRIME NTLA-2002 have been designated ODD & RMAT by FDA and Innovation Passport by UK MHRA

EDIT-301
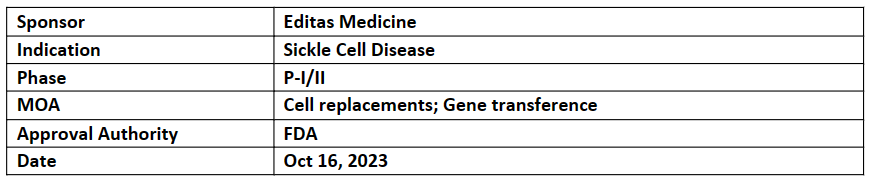
-
Editas’ EDIT-301 received RMAT from FDA for the treatment of severe SCD. Earlier in 2023, EDIT-301 also received ODD and RPDD for the treatment of SCD and beta thalassemia
-
EDIT-301 (single administration) is presently being investigated in the single-arm, open-label, multi-center P-I/II (RUBY) trial for its safety and efficacy to treat severe sickle cell disease
IMA203

-
Immatics’ IMA203 TCR-T received RMAT designation from FDA’s CBER for multiple r/r HLA-A*02:01 +ve & PRAME-expressing cancers (incl. cutaneous melanoma, uveal melanoma, endometrial carcinoma, synovial sarcoma, & ovarian cancer).
-
ACTengine IMA203 TCR-T is being assessed under 3 ongoing P-Ib studies with dose expansion in las-line solid tumor patients (here IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor):
-
Cohort A GEN1 monotherapy,
-
Cohort B in combination with an immune checkpoint inhibitor, and
-
Cohort C CD8 GEN2 monotherapy
-
IMA203 is a TCR-T cell therapy targeting PRAME, a protein frequently expressed in a large variety of solid tumors
-
FDA has awarded an RMAT designation to 92 products whereas received 238 requests as of Sep 30, 2023
Related Post: New Drug Designations - September 2023
Tags

Ritika is a content writer at PharmaShots. She is interested in covering recent innovations from pharma & medtech industry. She covers news related to Product approvals, clinical trial results, and updates. She is passionate, meticulous, diligent, and inquisitive. She can be contacted at connect@pharmashots.com.














